Substitution in general is the replacement of one functional group by another. It has been observed that tertiary alcohols react rapidly with HBr to give tertiary alkyl bromides. Primary alcohols, on the other hand, react very slowly with HBr and are usually converted to primary alkyl bromides when they react with PBr3.
How this difference in reactivities between tertiary and primary alcohols can be explained when undergo nucleophilic substitutions?
Note: A tertiary alcohol is one where the functional group –OH is attached to a carbon atom that it is connected with 3 carbon atoms. A primary alcohol is one where the –OH group is attached to a carbon atom that it is connected with 1 carbon atom.
 |
| Fig. 1: Reactivities of primary and tertiary alcohols with a Br- nucleophile (nucleophilic substitution) |
If we check the structure of those alcohols that react rapidly with HBr to give good yields of alkyl bromides, we find that all form stable carbocations– cations where the positive charge is on the carbon atom (Fig. 2):
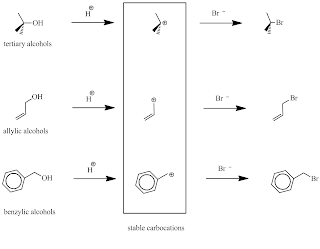 |
| Fig. 2: Stable carbocations formed during the reaction of tertiary, allylic or benzylic alcohols with HBr |
Reaction of secondary or tertiary alcohols with nucleophiles generally gives racemic products. When (+)-(S)-butan-2-ol reacts with SOClF/SbF5 and the resulting cation reacts with water the alcohol is regenerated but without any optical activity. Water has attacked the two faces of the planar cation with exactly the same probability and the final product is a 50-50 mixture of (S)-butanol and (R)-butanol – a racemic mixture (Fig. 3):
| Fig. 3: Reaction of the optically active (+)-(S)-butan-2-ol reacts with SOClF/SbF5 gives a (50% - 50%) mixture of (+)-(S)-butan-2-ol and (-)-(R)-butan-2-ol. The mixture is optically inactive. |
How the reaction of an optically active alcohol with a nucleophile can produce a racemic mixture?
Kinetic studies have shown that the reaction of tertiary alkyl halides (RX) with nucleophiles is first order and depends only on the concentration of the alkyl halide:
Rate of reaction = k . [RX]
The same is observed for the reaction of tertiary alcohols (ROH) with nucleophiles.
Reaction of the alkyl iodide RI (where R = C(CH3)3CH2-) with OH-/H2O or H2O yields different alcohols as products and an alkene (Fig.4). The products of the second reaction – where H2O acts as a nucleophile – have a carbon skeleton that is different than the carbon skeleton of the alkyl halide. A rearrangement of C atoms takes place. Since it has been proved that carbocations are prone to rearrangements it is suspected that a carbocation is an intermediate species:
When a strong nucleophile such as OH- is used instead of H2O the only reaction product of the alkyl halide RI (where R = C(CH3)3CH2-) is an alcohol and there is no any rearrangement of atoms. Another mechanism for this substitution reaction has been proposed which is called SN2 (substitution nucleophilic bimolecular).
What reaction mechanism can explain all the above evidence mainly for the reaction of tertiary alcohols or tertiary halides with nucleophiles (or even for secondary or primary halides with bulky substituents on the C atom such as the one shown in Fig.4) ?
The mechanism proposed for the nucleophilic substitutions that undergo tertiary alcohols or halides is called SN1 (substitutional nucleophilic unimolecular) and consists of two steps:
- Step 1: Slow ionization of the substrate (rate determining step)
- Step 2: Reaction of the carbocation with the nucleophile (Fig. 4)
 |
| Fig. 5: Mechanism of the Sn1 reaction |
The above proposed mechanism does account for the following:
- Tertiary alcohols or halides react faster than secondary and much faster than primary with nucleophiles under mild conditions (Fig. 1):
The rate determining step in the Sn1 mechanism is the formation of the carbocation. Stable carbocations are formed faster than less stable since the energy requirement ΔG is lower. Therefore, tertiary bromides are expected to react faster in an SN1 type reaction than primary bromides since when ionize give tertiary cations that are more stable and are produced faster than primary. It has been shown experimentally that the rate constant of simple alkyl bromides in SN1 type reactions increase as follows:
alkyl bromide | CH3Br | CH3CH2Br | (CH3)2CHBr | (CH3)3CBr |
type | methyl | primary | secondary | tertiary |
k1 (s-1) | 0.6 | 1.0 | 26 | 108 |
Table 1: Rate constants of simple alkyl bromides in SN1 type reactions
The stability of the corresponding carbocations increase in the same order (see post “Carbocations: Factors affecting Stability”).
- When an optically active substrate undergoes an SN1 type reaction a racemic mixture is formed (Fig. 3)
This experimental observation is consistent with the proposed mechanism (Fig. 5) that involves a carbocation. The intermediate species the carbocation – electron deficient species - is trigonal planar with an empty p orbital. The nucleophile can attack the carbocation from both sides (see Fig. 3) with equal probabilities giving a (50% - 50%) mixture of the R and S product and therefore an optically inactive product.
The fact that a carbocation is involved in SN1 type reactions is proved by the fact that compounds that have C atoms where a carbocation is not formed - such as at the bridgehead positions of [2.2.1](norbornyl) systems - do not react with nucleophiles:The carbocation in these cases is not formed since it cannot acquire a planar structure1-2.
References
1. P.D. Bartlett et. Al., J.A.C.S., 61, 3184 (1939)
2. G.A. Kraus et al., J. Org. Chem., 50, 4605 (1985)

 systems with substituents at the bridgehead position do not undergo SN1 type reactions since the corresponding carbocation is not formed since it cannot acquire a planar structure Fig. 6: [2.2.1](norbornyl) systems with substituents at the bridgehead position do not undergo SN1 type reactions since the corresponding carbocation is not formed since it cannot acquire a planar structure](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhE7nCBNdkYYR-Sy0r_5At1pPHpnaZA9JT7BfCl7_-fSmhTXnSCAKcY3Ev7GekFg8WgHjX3sNNHx606xXhXdPqCXh9pLmNlaA-t9yvXAfM2L7JoCuHE0dfBEDFKyQu0LnknavI42NzJnQuo/s400/norbornyl_sn1.png)
Hiç yorum yok:
Yorum Gönder